A mourner sniffs ammonia-soaked cotton wool handed out by medical rescue teams to help prevent fainting as she waits to pay her respects to Thailand's late King Bhumibol Adulyadej at the Grand
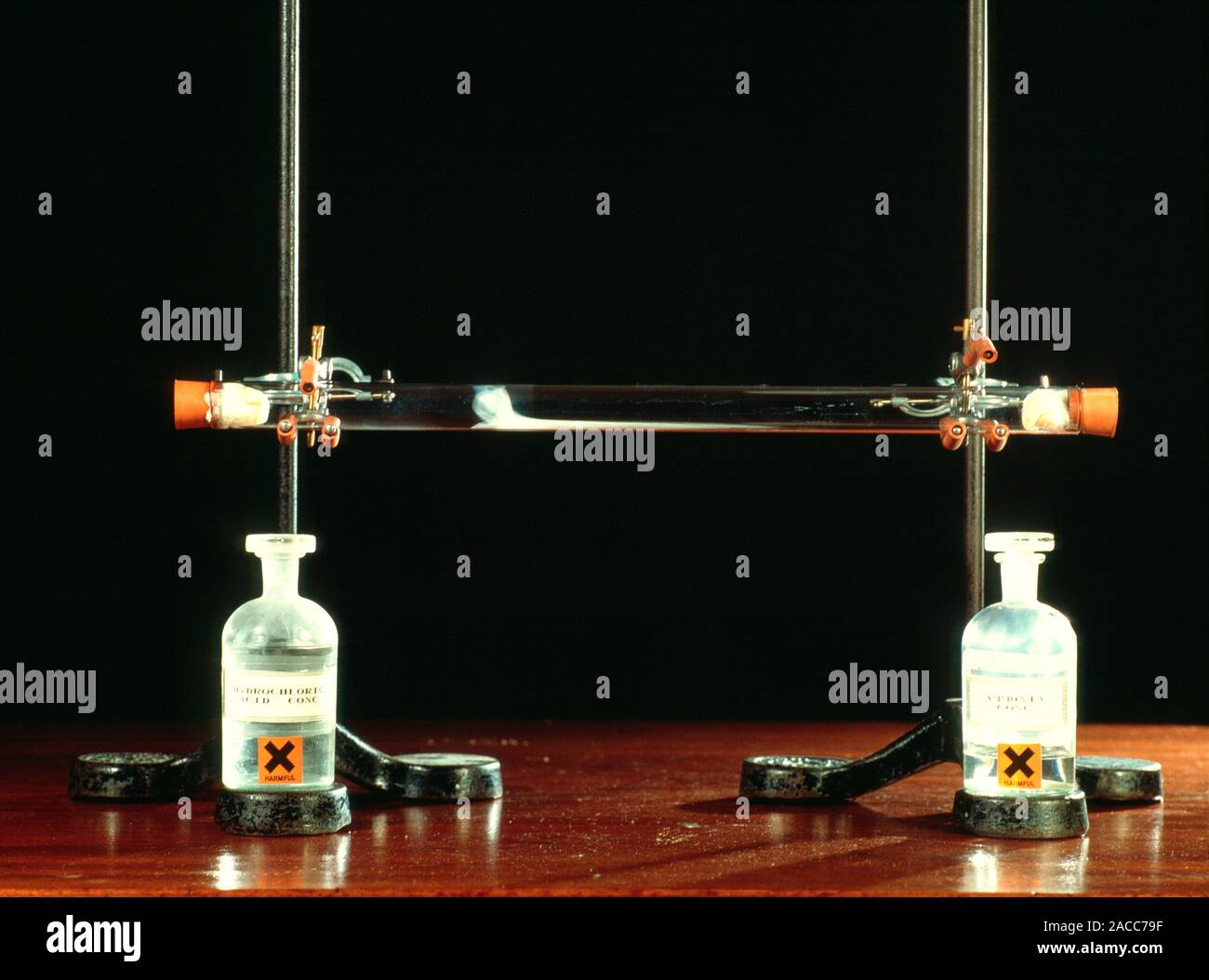
Ammonium chloride formation. Cotton wool balls have been soaked in hydrochloric acid (HCl, left) and ammonia (NH3, right). They have been placed at ei Stock Photo - Alamy

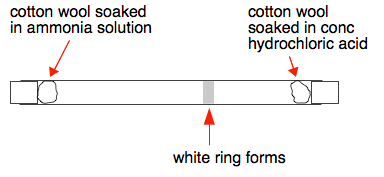
SOLVED: A piece of cotton wool soaked in Ammonia solution is placed at one end of a glass tube and at the same time another piece of cotton wool soaked in concentrated

A bottle of perfume is opened in the room why can we smell it after a while This is called diffusion Diffusion is when particles move from a high. - ppt download

In a gaseous reaction between ammonia and hydrochloric acid (HCl), a white precipitate of ammonium chloride is produced according to the following reaction. NH3(g) + HCl (g) rightarrow NH4Cl(s) Two cotton plugs





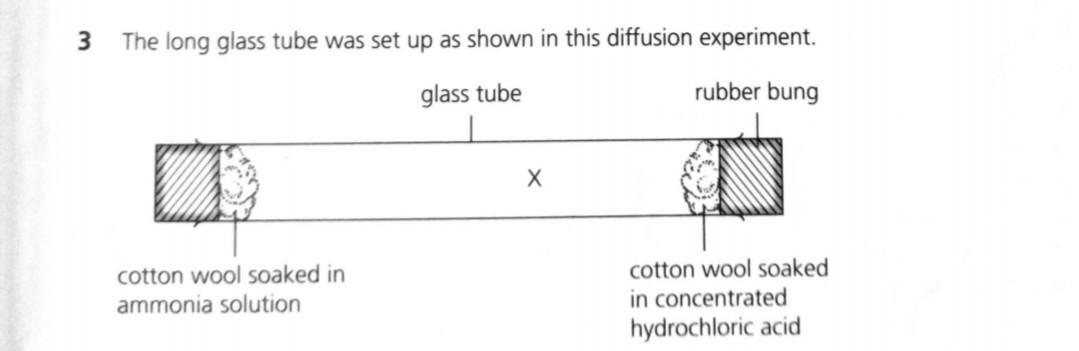



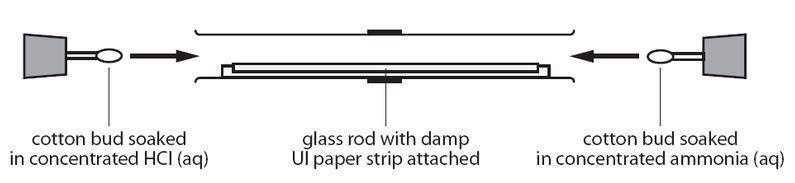



![Solved] The apparatus shown below was set up. Giv | SolutionInn Solved] The apparatus shown below was set up. Giv | SolutionInn](https://s3.amazonaws.com/si.question.images/images/question_images/1629/7/9/1/5716124a5537b1dc1629791571021.jpg)