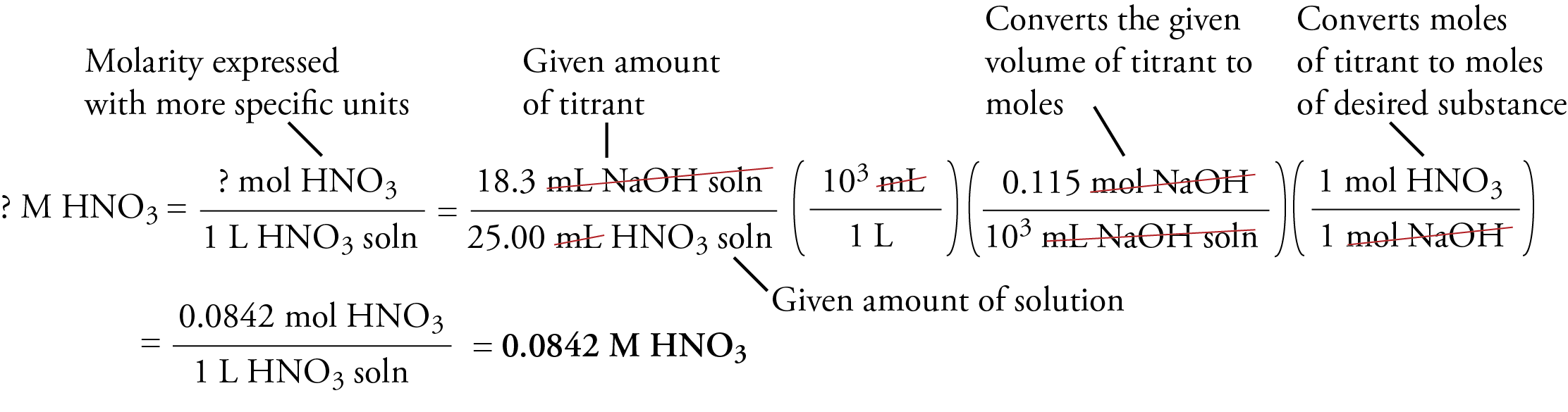
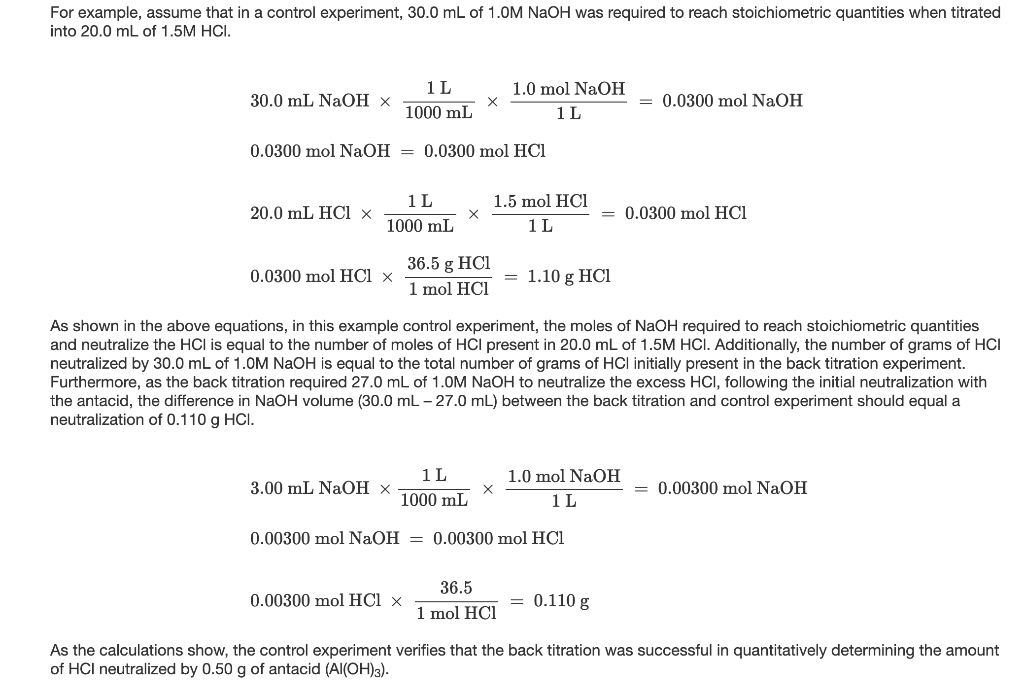
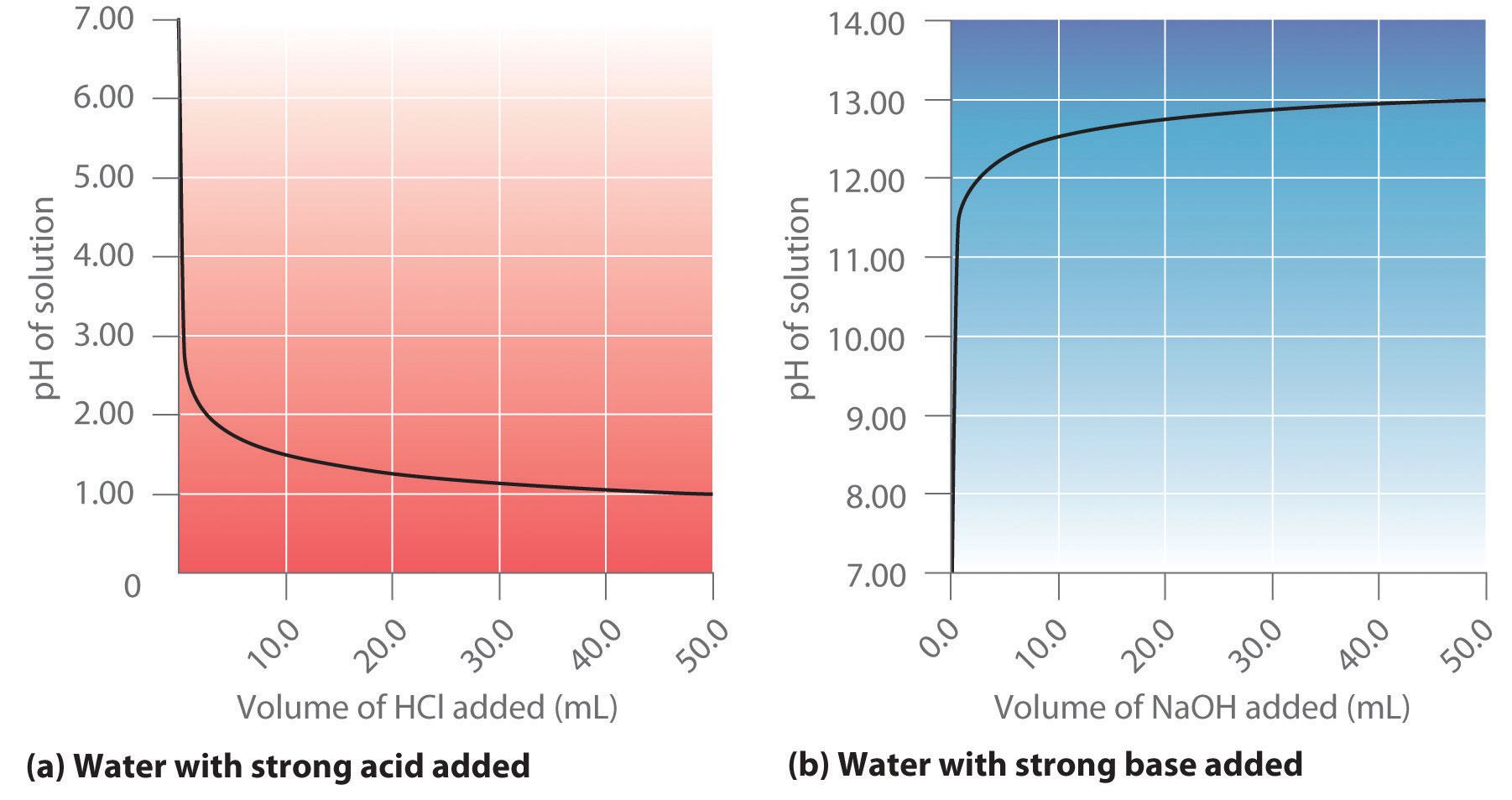
Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.

Number of moles of `NaOH` required for complete neutralization of `H^(+)` in solution which is form - YouTube

Enthalpy of neutralisation of acetic acid by NaOh is -50.6 kJ mol^(-1). Calculate DeltaH for ionisation of CH(3)COOH. Given. The heat of neutralisation of a strong acid with a strong base is -

Example 1 How many mL of M NaOH will completely neutralize 100 mL M H2SO4? - ppt video online download
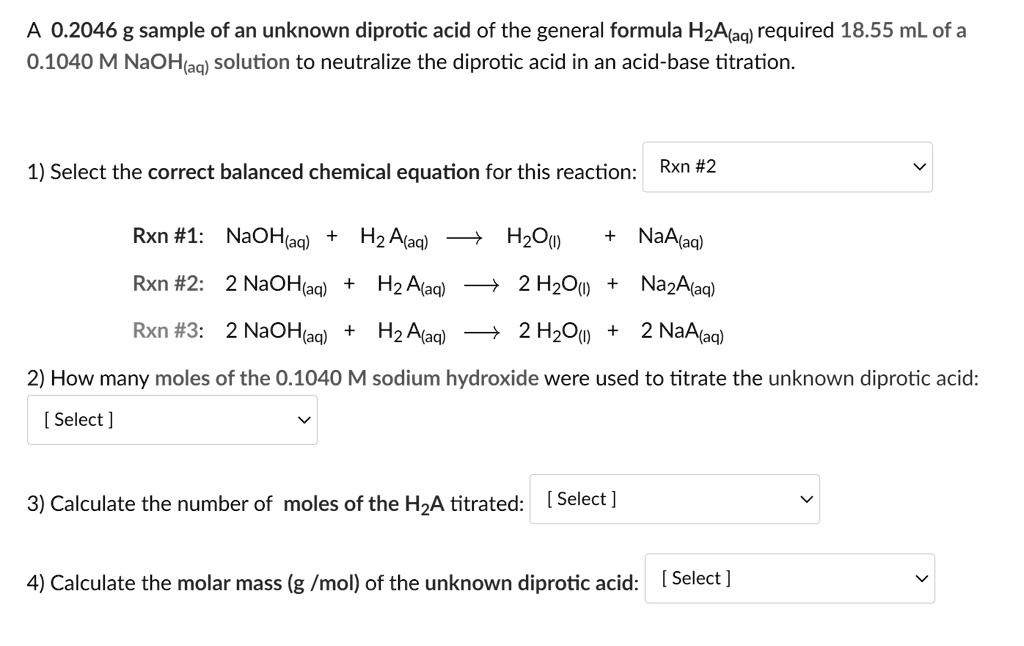
SOLVED: A 0.2046 g sample of an unknown diprotic acid of the general formula HzAlaq) required 18.55 mL of a 0.1040 M NaOHiaq) solution to neutralize the diprotic acid in an acid-base
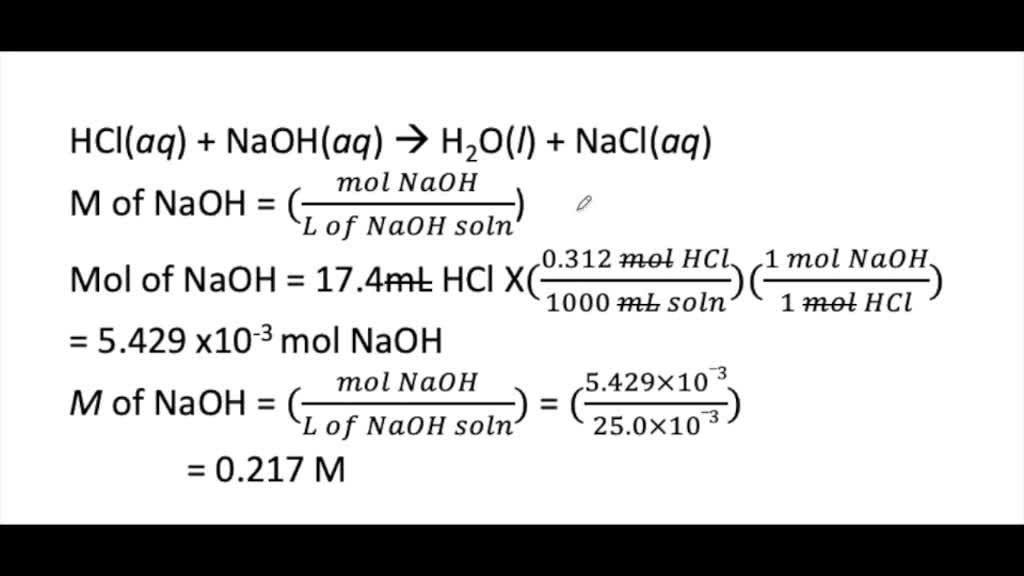
SOLVED:Calculate the concentration (in molarity) of an NaOH solution if 25.0 mL of the solution is needed to neutralize 17.4 mL of a 0.312 M HCl solution.
Calculate the volume of 1.00 mol L^ -1 aqueous sodium hydroxide that is neutralized by 200 mL of 2.00 mol L^-1 aqueous hydrochloric acid - Sarthaks eConnect | Largest Online Education Community