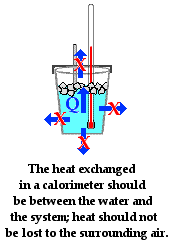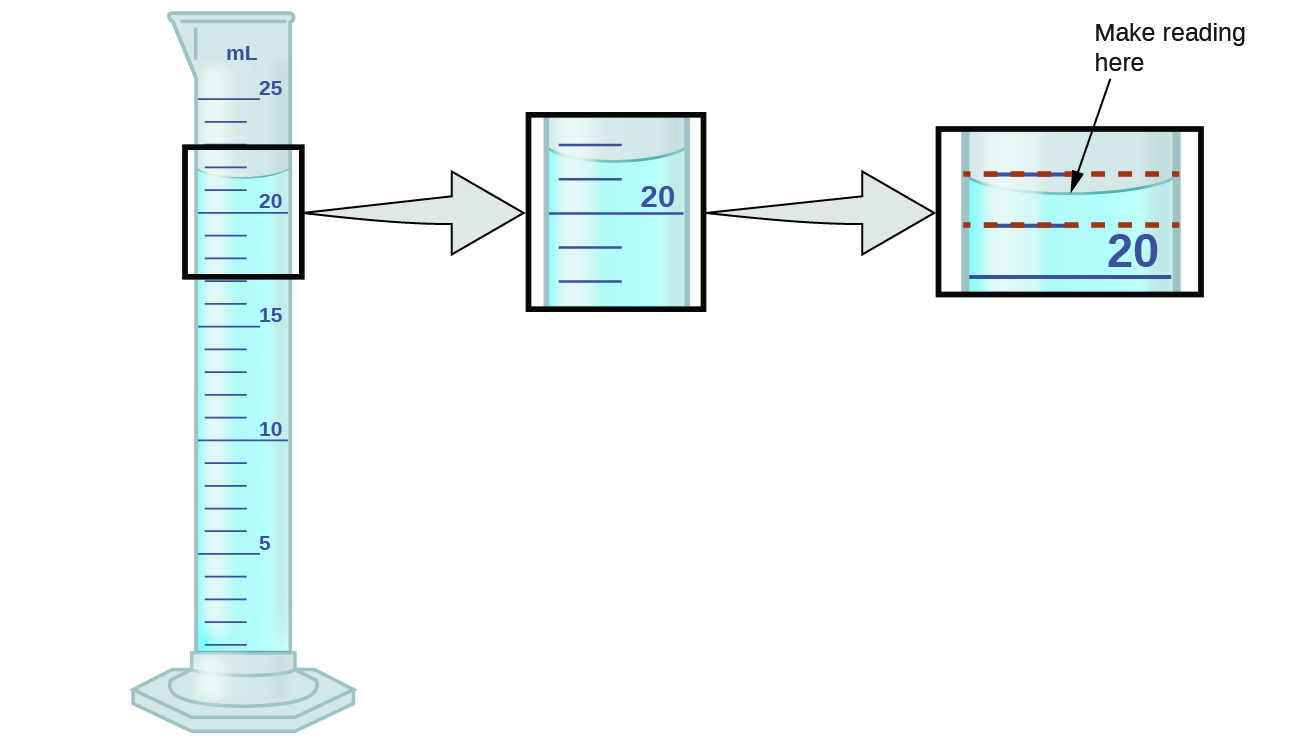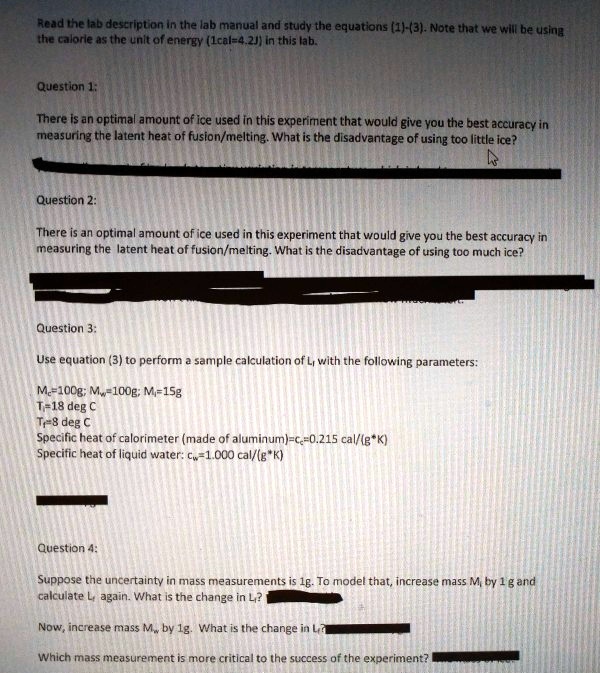
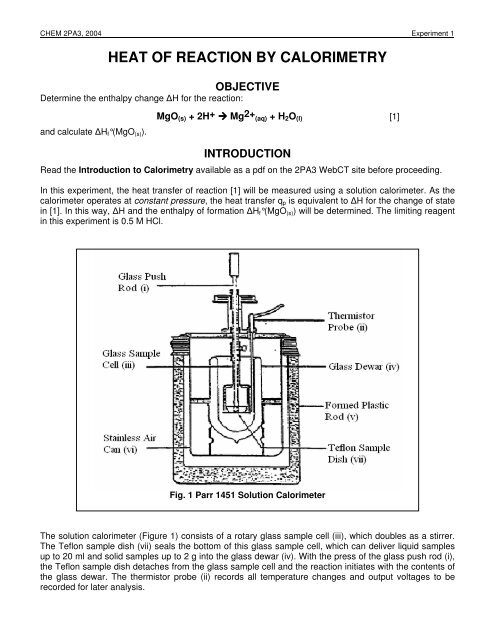
SOLVED: Read the lab descripton In the lab manual and study the equations (1)-(3. Note that we will be usina the calorle #s thc unlt of energy (Lcal-4.2J) In this lab: Question

Calorimetry Problems, Thermochemistry Practice, Specific Heat Capacity, Enthalpy Fusion, Chemistry - YouTube

Seminar assignments - Specific heat and heat of fusion questions - Questions 1. What do you see as - Studocu

A level Experimental methods for determining enthalpy changes treatment of results bomb calorimeter sources of error graphical analysis KS5 GCE chemistry revision notes
How to calculate the uncertainty of C heat capacity in the following formula knowing the measure and uncertainty to M1, M2, T1, T2 ? M1 c1 (T1- Tf) = m2 c2 (Tf -

MJ14 P12 Q5 Percentage Uncertainty in Temperature Change | May/June 2014 | CAIE A Level 9702 Physics - YouTube







![11.1 Determine the uncertainties in results [SL IB Chemistry] - YouTube 11.1 Determine the uncertainties in results [SL IB Chemistry] - YouTube](https://i.ytimg.com/vi/B7dKWE-0FZY/maxresdefault.jpg)