which ion has maximum magnetic moment? 1)Fe^3+ 2)Mn^2+ and why because both has same no of unpaired electron
Which of the following ion has maximum magnetic moment ? 1.Mn^3+ 2.Cu^2+ 3. Fe^3+ 4.v^3+ Also explain what is magnetic moment ?

First principles calculation of magnetic order in a low-temperature phase of the iron ludwigite - ScienceDirect

Illustration of the net magnetic moment calculation in various types of... | Download Scientific Diagram
![Explain why [Fe(H(2)O)(6)]^(3+) has magnetic moment value of 5.92 BM where as [Fe(CN)(6)]^(3-) has a value of only 1.74 BM ? Explain why [Fe(H(2)O)(6)]^(3+) has magnetic moment value of 5.92 BM where as [Fe(CN)(6)]^(3-) has a value of only 1.74 BM ?](https://d10lpgp6xz60nq.cloudfront.net/ss/web/584381.jpg)
Explain why [Fe(H(2)O)(6)]^(3+) has magnetic moment value of 5.92 BM where as [Fe(CN)(6)]^(3-) has a value of only 1.74 BM ?
Amongst the following ions which one has the highest magnetic moment value? (i) [Cr(H2O)6]3+ (ii) [Fe(H2O)6]2+ - Sarthaks eConnect | Largest Online Education Community
Calculate the magnetic moments of the following complexes : (i) [Fe(CN)6]-4 (ii) [FeF6]-3 - Sarthaks eConnect | Largest Online Education Community
![The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are](https://d10lpgp6xz60nq.cloudfront.net/ss/web/1263733.jpg)
The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are
![Explain why [Fe(H 2 O) 6 ] 3+ has high magnetic moment value of 5.92 BM whereas magnetic moment of [Fe(CN) 6 ] 3– has value of only 1.74 BM. Explain why [Fe(H 2 O) 6 ] 3+ has high magnetic moment value of 5.92 BM whereas magnetic moment of [Fe(CN) 6 ] 3– has value of only 1.74 BM.](https://static.insightsonindia.in/ncertusercontent/solutions/?domain=gF&l=PROJ38513/156889677077182.jpg)

![The spin magnetic moment of iron in K3 [ Fe (CN)6 ] is : The spin magnetic moment of iron in K3 [ Fe (CN)6 ] is :](https://haygot.s3.amazonaws.com/questions/1952807_1117559_ans_880cbb7d24bb4d01ab49a890bc90ed14.jpg)





![Calculate magnetic moment of Fe^3 + in [Fe(CN)6]^3 - and in [Fe(H2O)6]^3 - . Calculate magnetic moment of Fe^3 + in [Fe(CN)6]^3 - and in [Fe(H2O)6]^3 - .](https://haygot.s3.amazonaws.com/questions/1576306_1732047_ans_78403cb9299c4e63a40902c700aaa8a9.jpg)


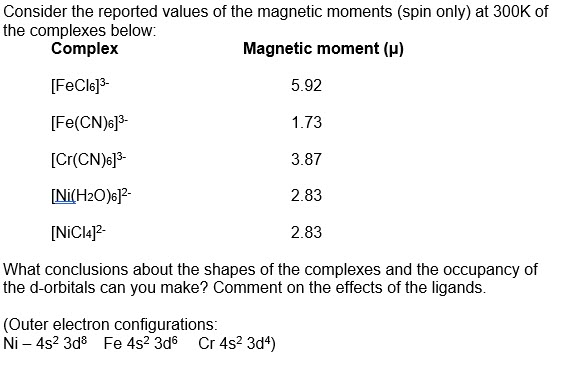

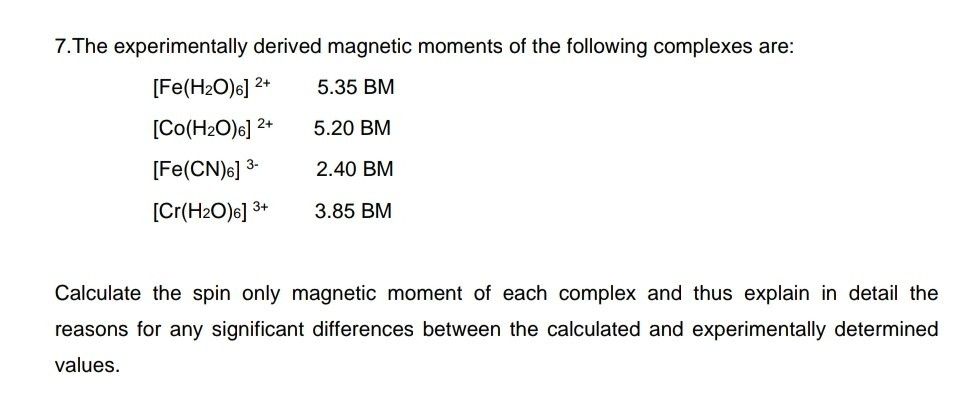
![6)]^(3+)` has magnetic moment value of 5.92 BM whereas `[Fe(CN) 6)]^(3+)` has magnetic moment value of 5.92 BM whereas `[Fe(CN)](https://i.ytimg.com/vi/h9yxC3M4DSM/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgVChVMA8=&rs=AOn4CLAIfOZpXbuvpM77ZlcUR5tTbbLZLA)