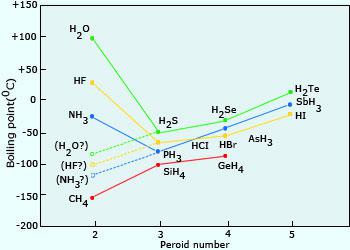
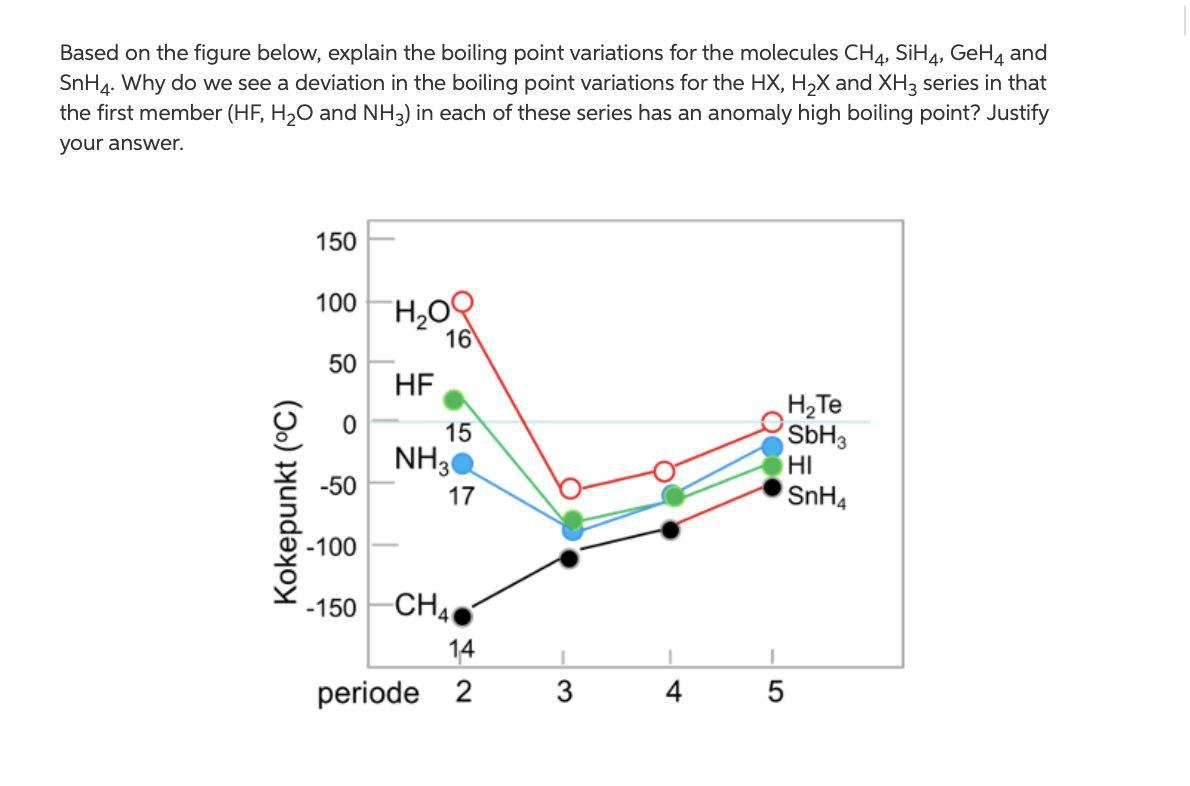
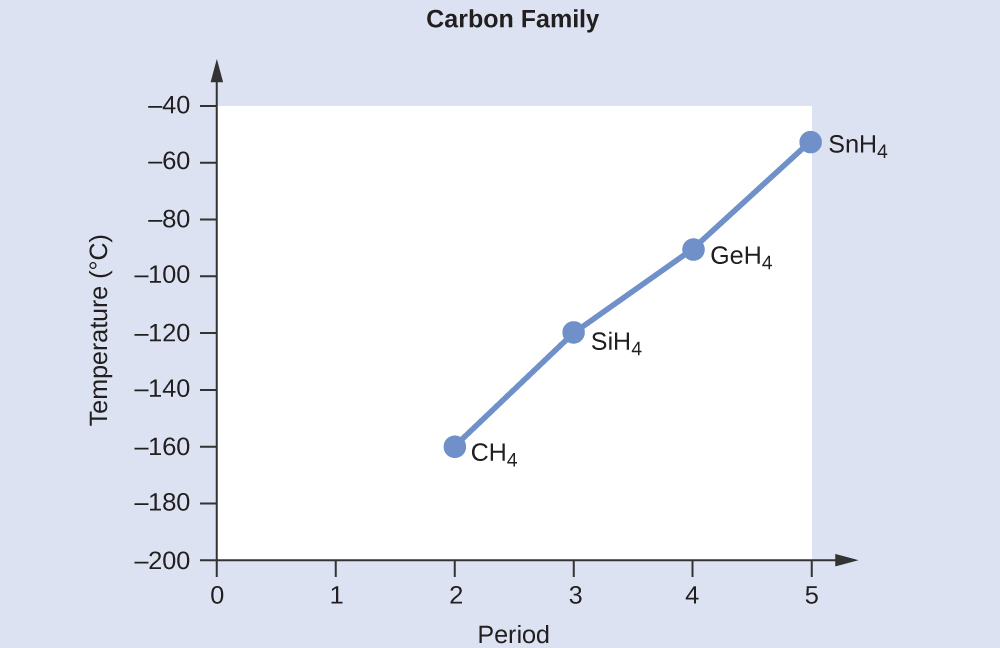
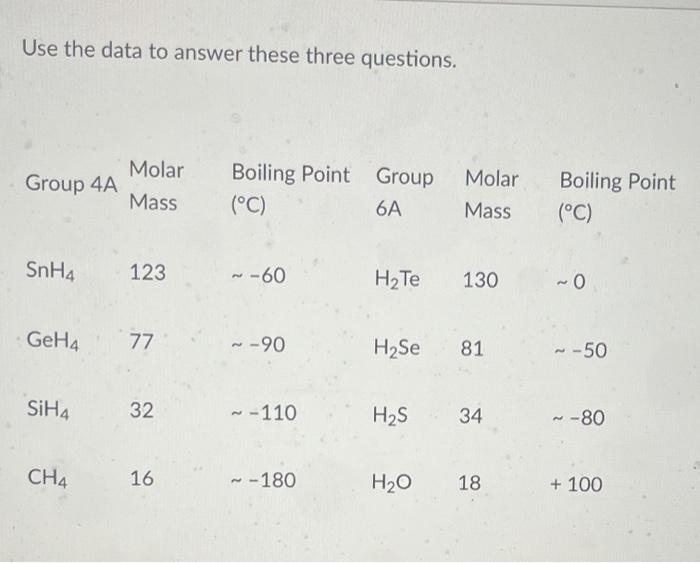
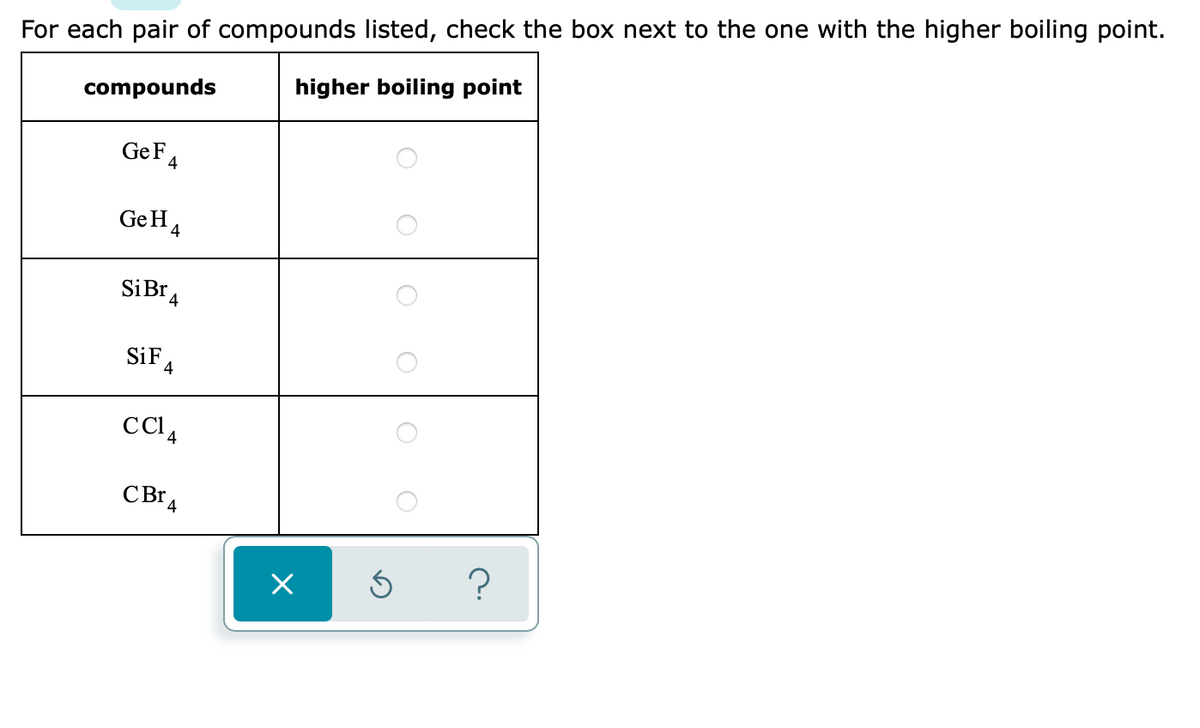
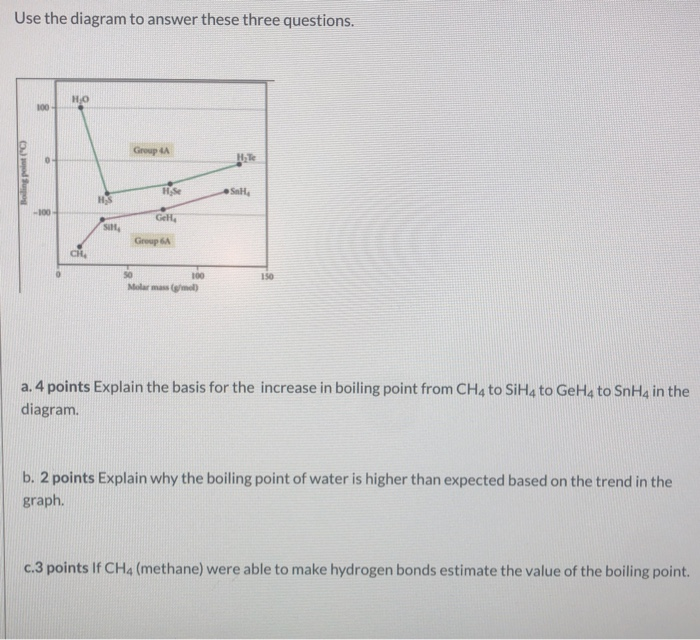
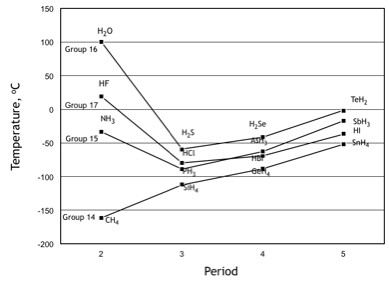
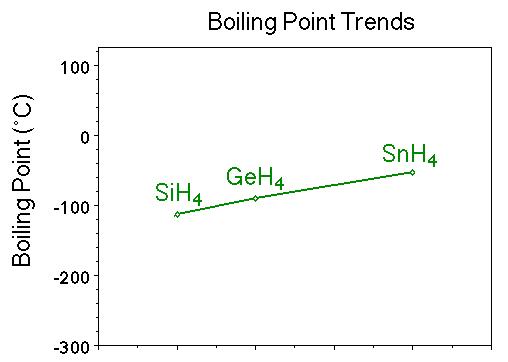
SOLVED:Estimate the missing boiling point in the following series of compounds. (a) CH4,-164^∘ C ; SiH4,-112^∘ C ; GeH4,-90^∘ C sn H4, ?^∘ C (b) H2 O, ?^∘ C ; H2 S,-61^∘
Consider the following: CH4, SiH4, GeH4, SnH4 The boiling points for these compounds increase roughly at the same rate except for CH4. Why does CH4 have a significantly lower boiling point than
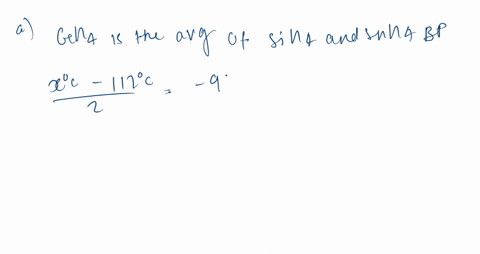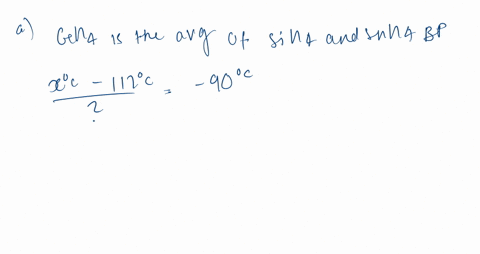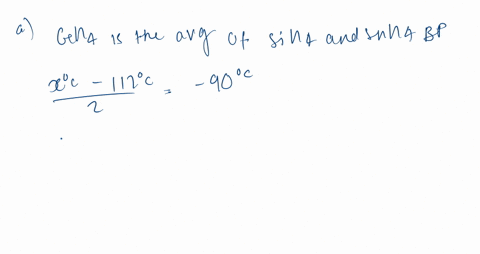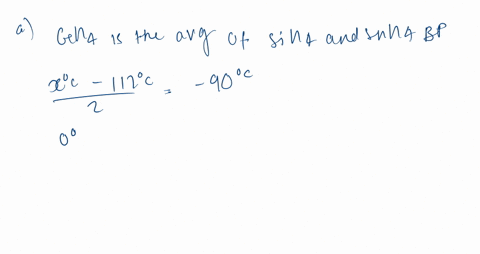
SOLVED:Estimate the missing boiling point in the following series of compounds. (a) CH4,-164^∘ C ; SiH4,-112^∘ C ; GeH4,-90^∘ C sn H4, ?^∘ C (b) H2 O, ?^∘ C ; H2 S,-61^∘