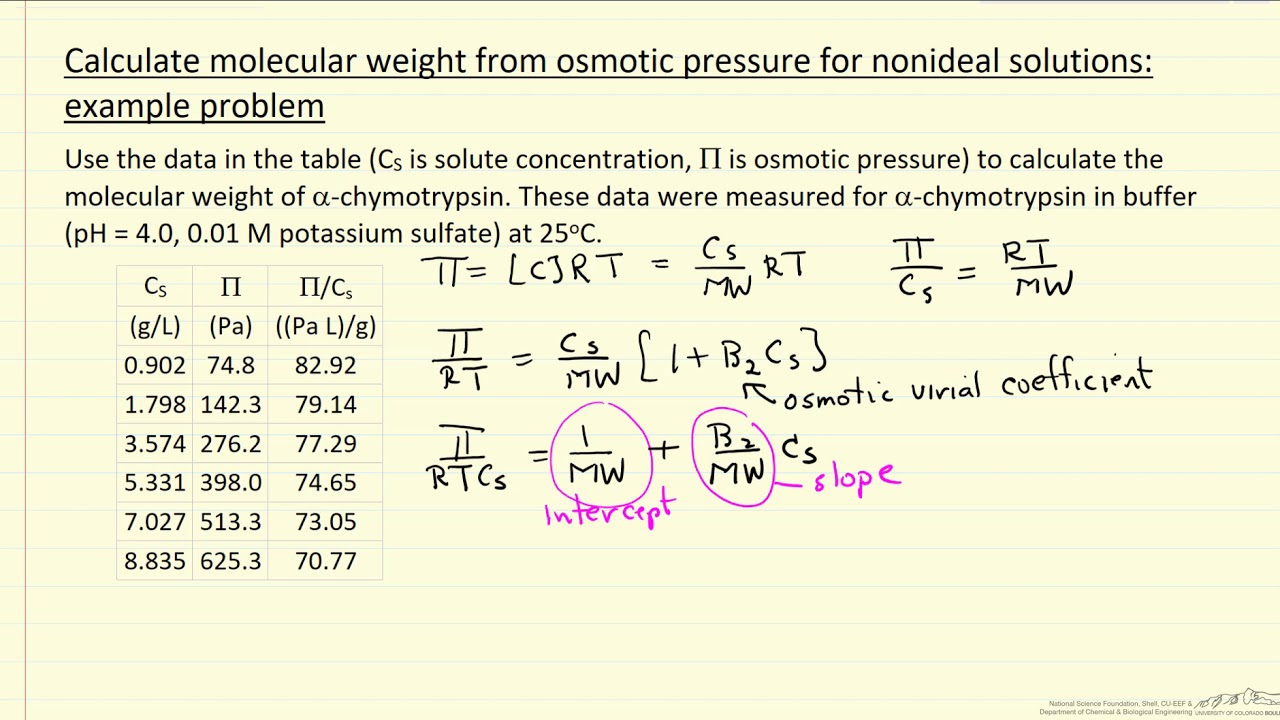
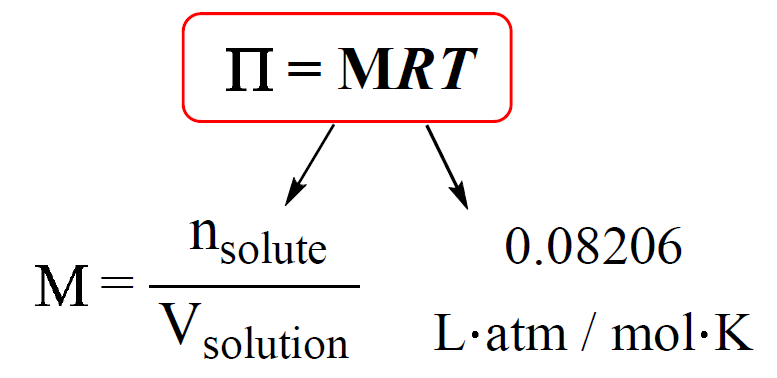
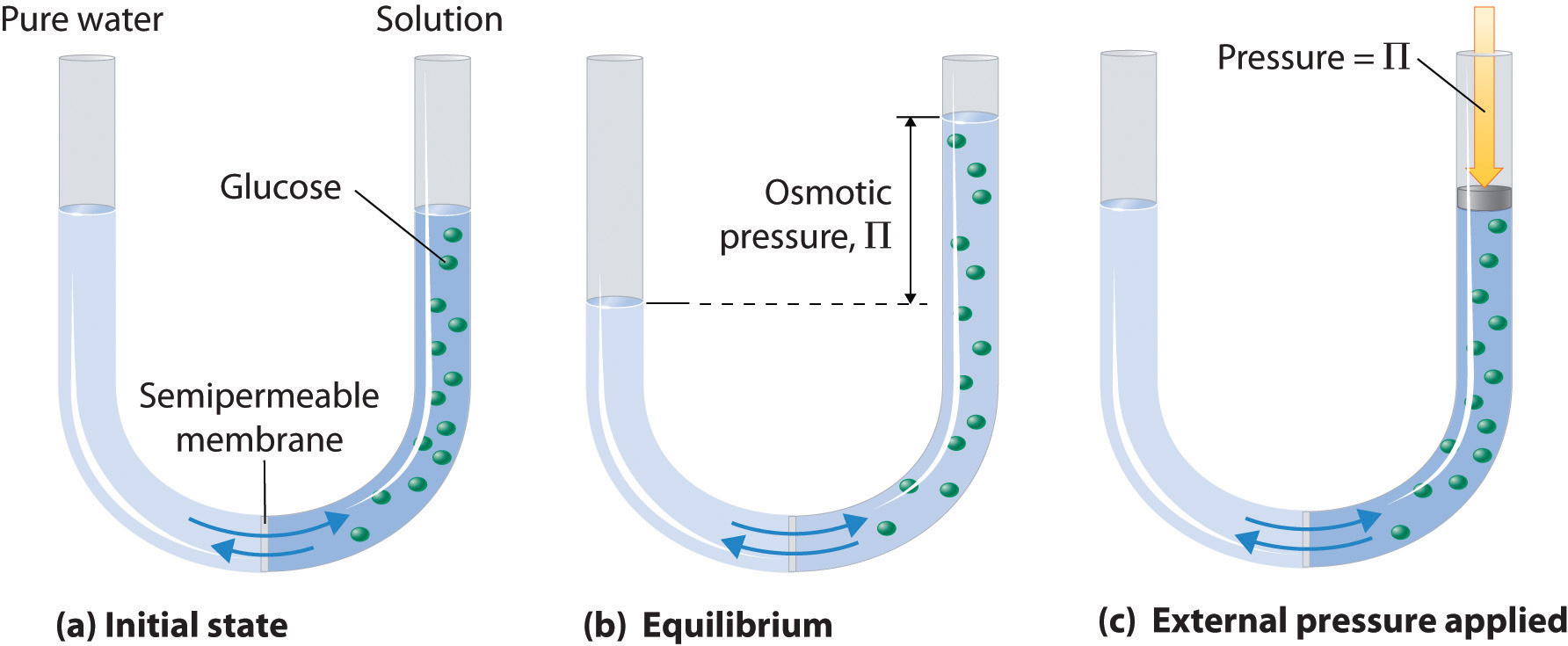
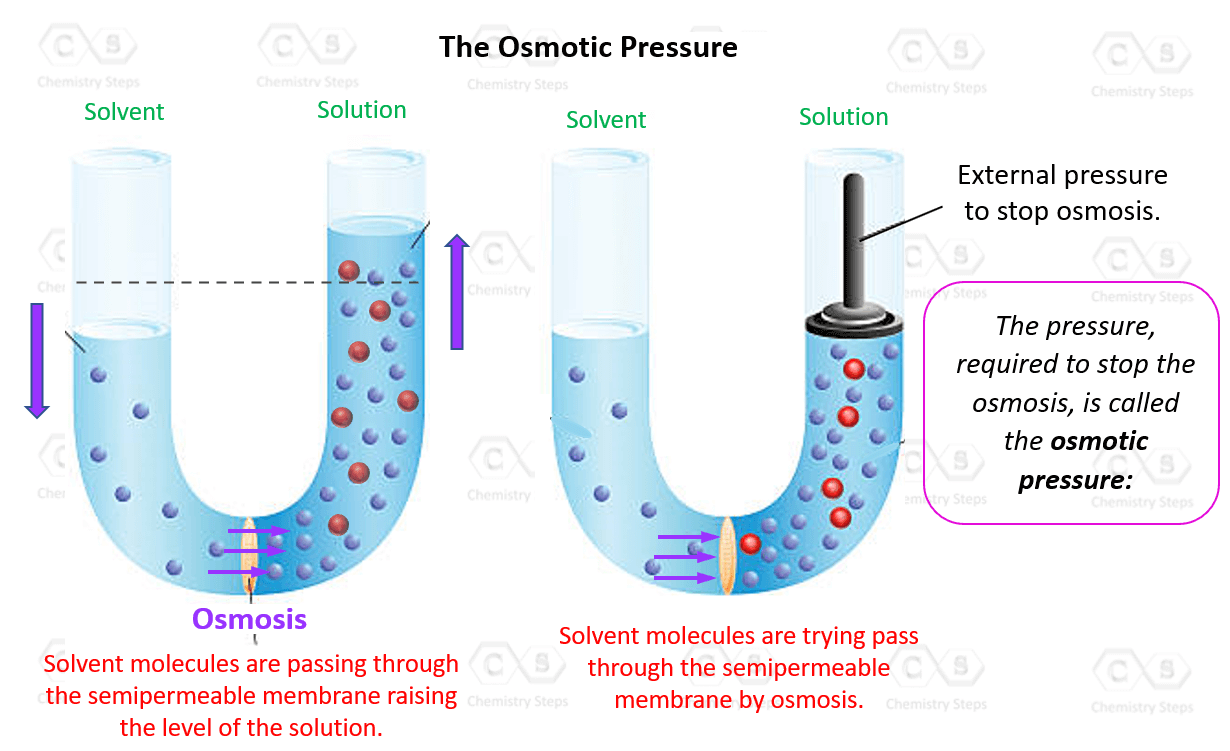
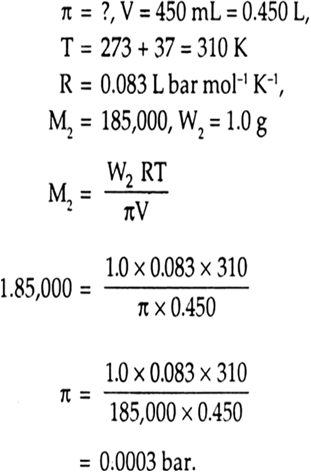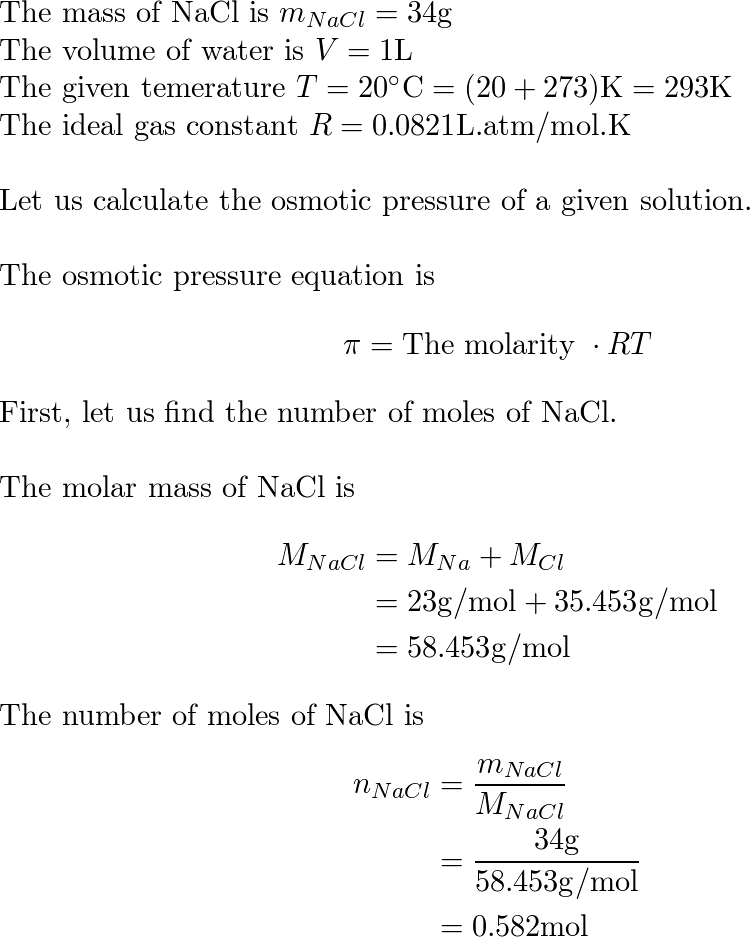The osmotic pressure of a solution (density is 1 g `mL^(-1))` containing `3 g` of glucose (molec... - YouTube
![Calculate the osmotic pressure exerted by a solution prepared by dissolving 1.5 g of a polymer of molar mass 185000 in 500 ml of water at 37^o C. [R = 0.0821 L.atm.K^-1.mol^-1 ] Calculate the osmotic pressure exerted by a solution prepared by dissolving 1.5 g of a polymer of molar mass 185000 in 500 ml of water at 37^o C. [R = 0.0821 L.atm.K^-1.mol^-1 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/UHRWMHREWDZsQ0k=/sd/)
Calculate the osmotic pressure exerted by a solution prepared by dissolving 1.5 g of a polymer of molar mass 185000 in 500 ml of water at 37^o C. [R = 0.0821 L.atm.K^-1.mol^-1 ]
![Calculate the osmotic pressure exerted by a solution prepared by dissolving 1.5 g of a polymer of molar mass 185000 in 500 ml of water at 37^o C. [R = 0.0821 L.atm.K^-1.mol^-1 ] Calculate the osmotic pressure exerted by a solution prepared by dissolving 1.5 g of a polymer of molar mass 185000 in 500 ml of water at 37^o C. [R = 0.0821 L.atm.K^-1.mol^-1 ]](https://i.ytimg.com/vi/PtV0tDX6lCI/maxresdefault.jpg)
Calculate the osmotic pressure exerted by a solution prepared by dissolving 1.5 g of a polymer of molar mass 185000 in 500 ml of water at 37^o C. [R = 0.0821 L.atm.K^-1.mol^-1 ]
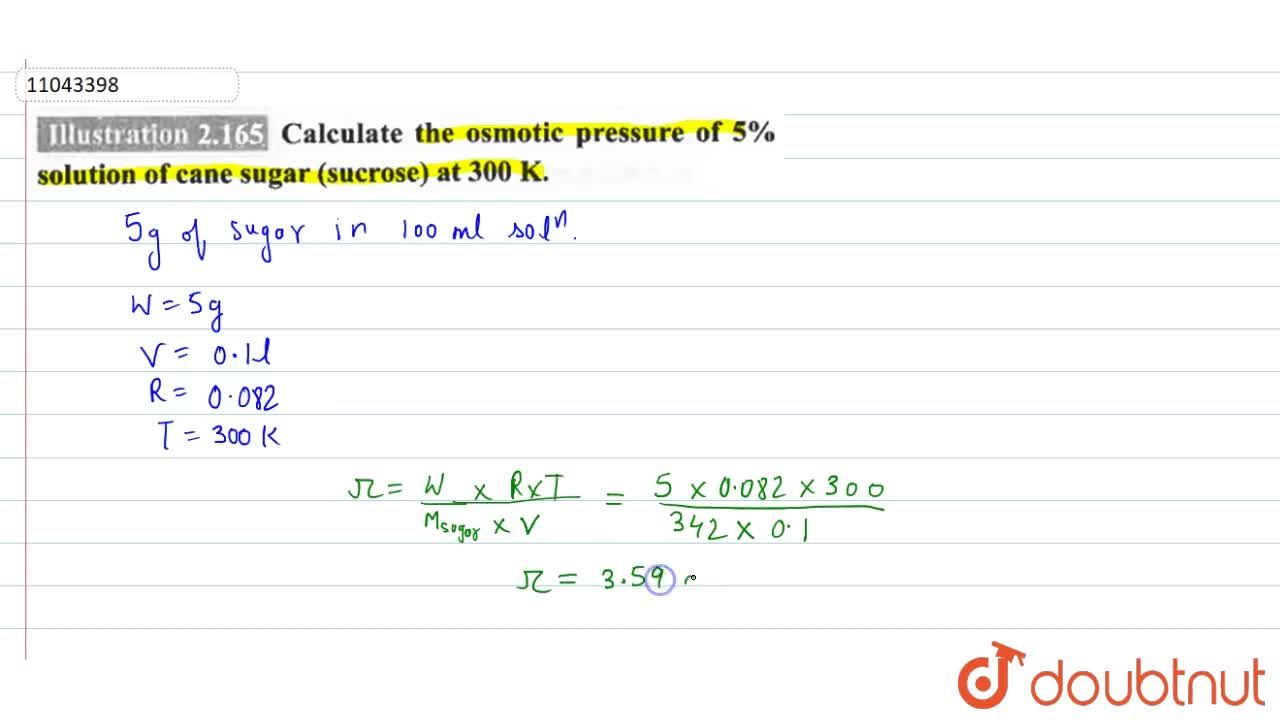
Calculate the osmotic pressure of 5% solution of urea at 273K. - Sarthaks eConnect | Largest Online Education Community
![For a 5% solution of urea (Molar mass - 60 g/mol), calculate the osmotic pressure at 300 K. [R = 0.0821 L atm K^(-1) mol^(-1)] For a 5% solution of urea (Molar mass - 60 g/mol), calculate the osmotic pressure at 300 K. [R = 0.0821 L atm K^(-1) mol^(-1)]](https://d10lpgp6xz60nq.cloudfront.net/ss/web/1522124.jpg)
For a 5% solution of urea (Molar mass - 60 g/mol), calculate the osmotic pressure at 300 K. [R = 0.0821 L atm K^(-1) mol^(-1)]