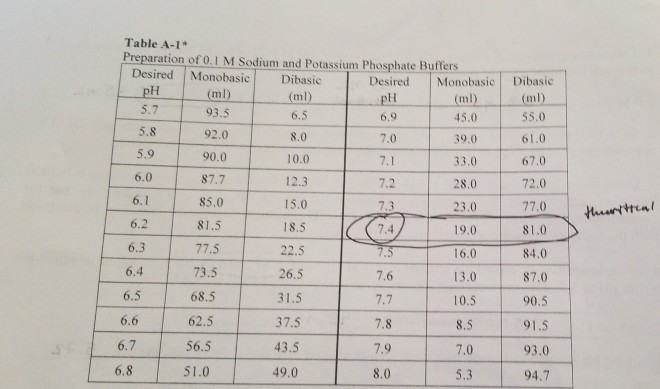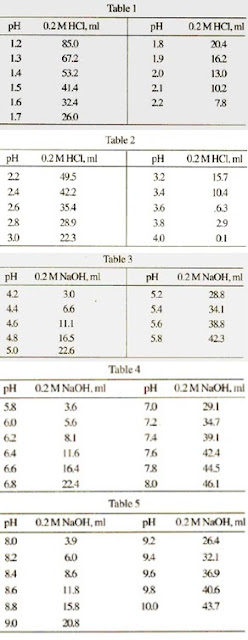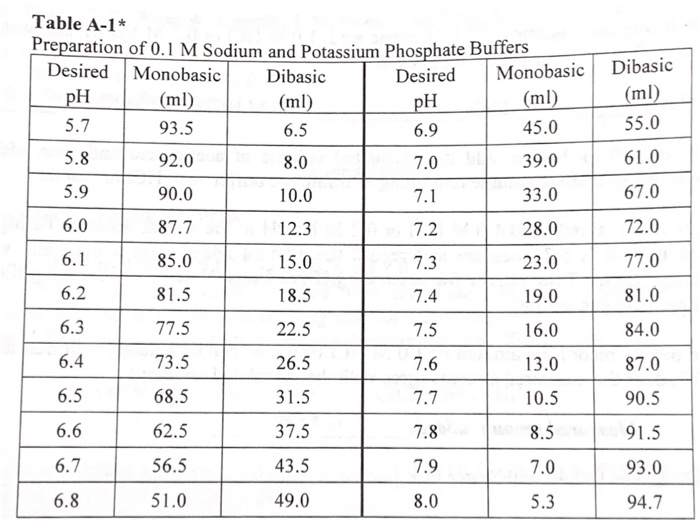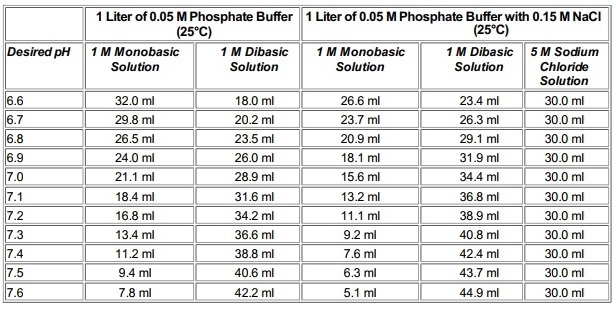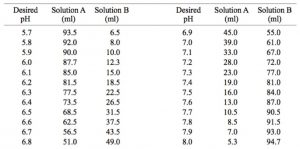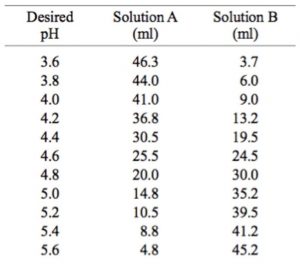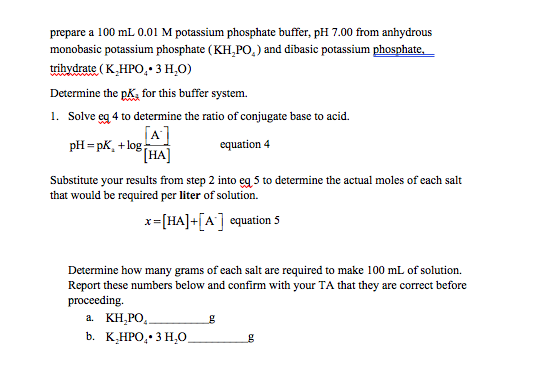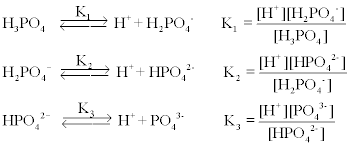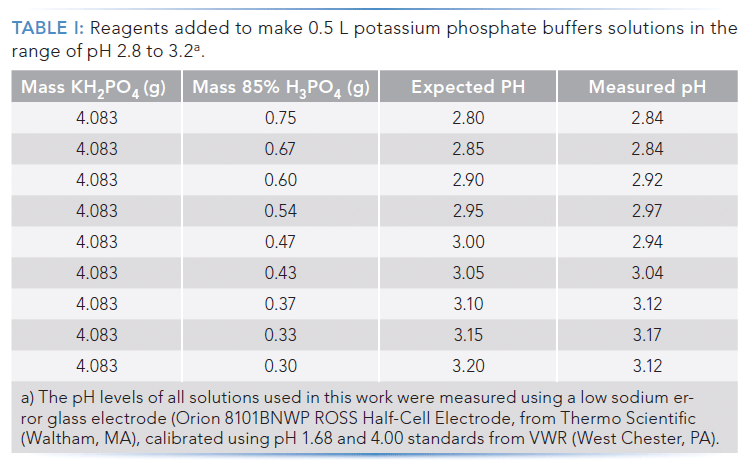
Mobile Phase Buffers in Liquid Chromatography (LC): Effect of Buffer Preparation Method on Retention Repeatability

Preparation of Buffers - 1 Calculate the volume of sulfuric acid (H 2 SO 4 ) necessary to prepare 600 milliliter 0.5M H 2 SO 4 from concentrated H 2 SO. - ppt download
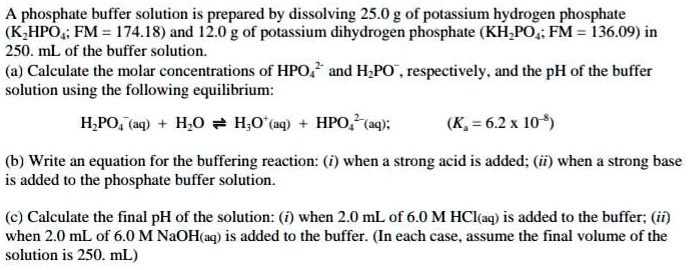
SOLVED: A phosphate buffer solution is prepared by dissolving 25.0 g of potassium hydrogen phosphate (K,HPO ; FM = 174.18) and 12.0 g of potassium dihydrogen phosphate (KH,PO : FM = 136.09)
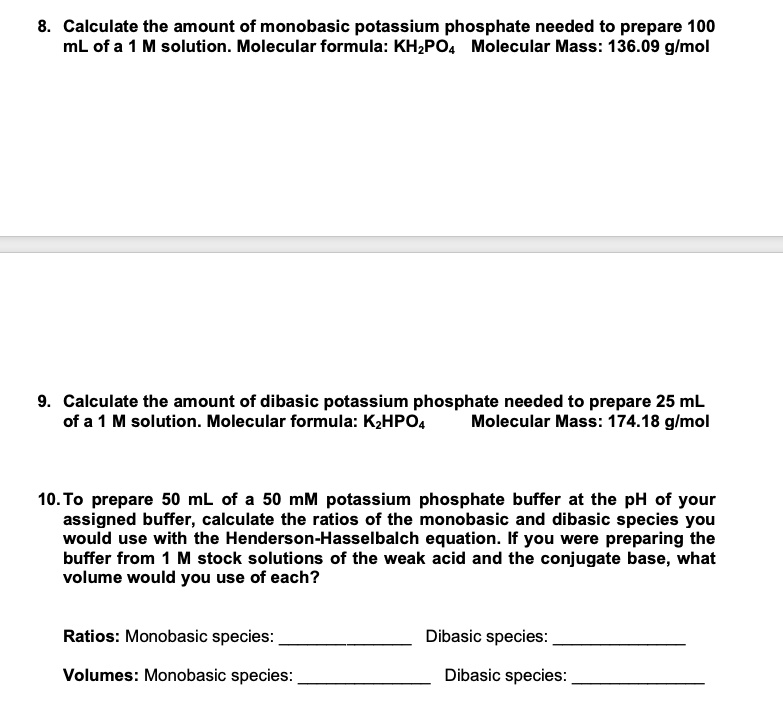
SOLVED: Calculate the amount of monobasic potassium phosphate needed to prepare 100 mL ofa M solution Molecular formula: KHZPOa Molecular Mass: 136.09 glmol Calculate the amount of dibasic potassium phosphate needed to