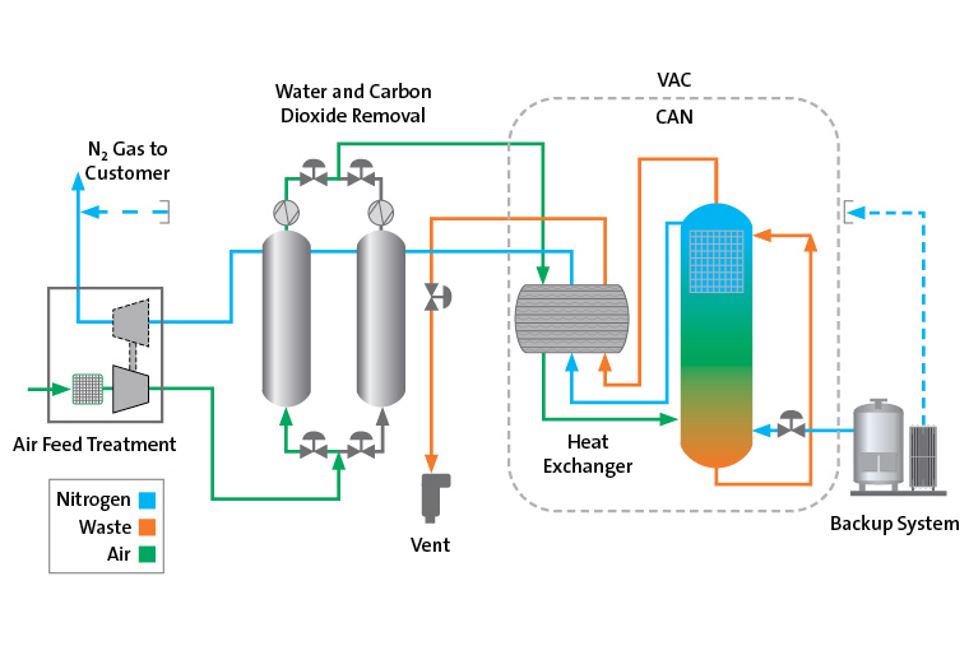
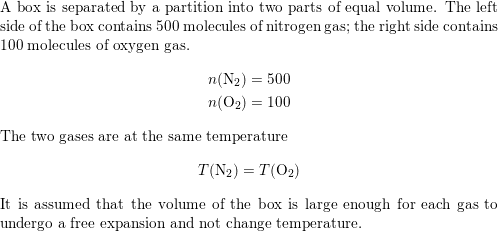IES Newsletter, volume 15 issue 6 - Cary Institute of Ecosystem Studies - New York Heritage Digital Collections

Two rigid boxes containing different ideal gases are placed on table. Box A contains one mole of nitrogen at temperature T0 , while box B contains 1 mole of helium at temperature
![Nitrogen gas is filled in a container of volume 2.32L at 32^oC and at 4.7 atm pressure. Calculate the number of moles of gas.[Write up to 3 decimal places] Nitrogen gas is filled in a container of volume 2.32L at 32^oC and at 4.7 atm pressure. Calculate the number of moles of gas.[Write up to 3 decimal places]](https://dwes9vv9u0550.cloudfront.net/images/4730475/db9edf02-73d5-4fdb-b5b6-bd216403235a.jpg)
Nitrogen gas is filled in a container of volume 2.32L at 32^oC and at 4.7 atm pressure. Calculate the number of moles of gas.[Write up to 3 decimal places]

SOLVED: 20.34 . A box is separated by a partition into two parts of equal volume. The left side of the box contains 500 molecules of nitrogen gas; the right side contains

Precision Nitrogen Oven/Precision N2 Box Oven, Precision Hot Air Oven Wholesaler - Precision Nitrogen Oven - Precision Nitrogen Oven
A rigid †an k contains 35kg of nitrogen at 6 atm . sufficient quantity of oxygen is supplied to increase pressure to 9atm. hile the temperature remains cons†an t. Amount of oxygen